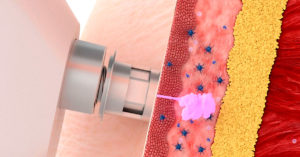
Although there are many delivery systems that facilitate the intradermal application of vaccines (Kis et al., 2012), in pigs, needle-free jet injectors are, without doubt, the most widely used (Figure 1). The intradermal application of vaccines with a needle-free injector involves the administration of small volume of vaccine of around 0.2 ml.

Figure 1. Hipradermic® 3.0 needle-free jet injector for intradermal application of vaccines.
There are many benefits of the intradermal application of vaccines with a needle-free jet injector, especially with regard to the non-use of needles, such as: elimination of the possibility of broken needles and damage to the carcass, or the risk of accidental needle stick injuries to employees.
They also reduce the risk of spreading viraemic disease such as PRRSv, which occurs when re-using the same needle-syringe on multiple animals.
In addition, needle-free vaccination through intradermal injection has many advantages compared to other types of vaccination, being a less invasive technique and causing less anxiety and pain to the animal.
Lowering the pain and stress of pigs in production has been shown to increase their general health and growth capabilities (Martínez-Miró et al., 2016).
Needle-free mechanism
The mechanism of needle-free jet injectors relies on the principle of forcing fluids through a small orifice, generating a high-pressure stream that penetrates into the skin with a high velocity (of approximately 100 m/s).
The impact of the fluid on the skin generates a hole through which the liquid is forced into the tissue without the help of a needle.
The delivery of a liquid formulation by a liquid jet injector can be divided into three general stages, requiring a total time of less than 0.3 seconds:
At first a peak pressure phase is observed, with optimal pressure used to penetrate the skin (Figure 2. Pressure profile: A).
This is followed by the dispersion phase. B), that provides a wide dispersion of the liquid into the tissue as it forces a path through low resistance regions, resulting in a widely dispersed, spider-web- like distribution of vaccine.
This wide dispersion of vaccine is thought to increase exposure of antigen to antigen presenting- cells, thereby resulting in an enhanced immune response.
Finally, there is the drop-off phase (Figure 2. Pressure profile: C).
The consistent pressure profile of a needle-free jet injector ensures a homogenous process where each animal is vaccinated at the selected tissue depth (Chase et al., 2008).
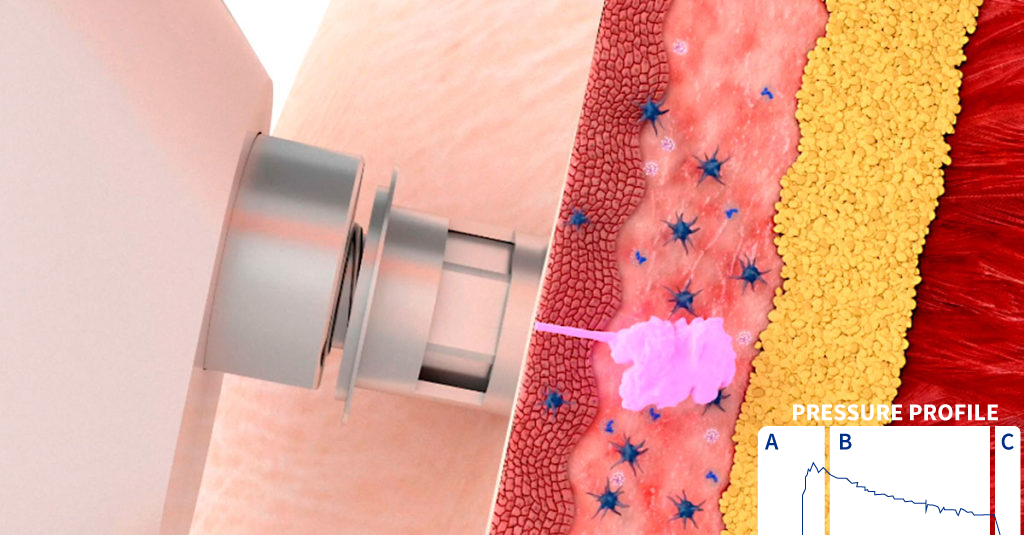
Figure 2. Representation of the three different pressure stages of a needle- free device during the intradermal administration of a vaccine into pig skin.
Vaccination: Intradermal application
In the case of the intradermal application of vaccines, it is important to adequately adjust the pressure and force of the needle-free device to ensure a deposit of the vaccine specifically at the dermis layer (Figure 3).
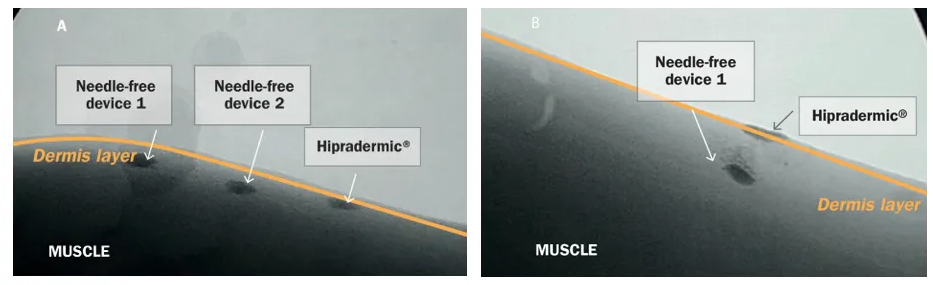
Figure 3. Intradermal and transdermal distribution of a specific vaccine in pig skin with different needle-free injector devices shown with real-time X-ray. Administration of the vaccine to the back region of the pig (A) and to the neck region of the pig (B). Internal study.
When depositing an intradermal vaccine outside the dermis layer the efficacy expected cannot be guaranteed.
Therefore, it is important to highlight that not all needle-free jet injectors available on the market may be able to perform with the same precision to ensure successful delivery of the vaccine at the dermis layer.
References: